TOXLAB -Pharmaceuticals
Regulatory Toxicology Services
Ensuring product safety and regulatory compliance is paramount in the pharmaceutical industry. TOXLAB offers comprehensive toxicological risk assessment services to address critical areas such as:
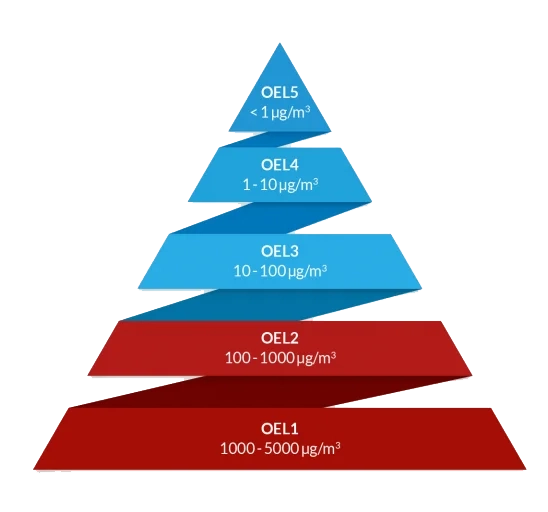
- Health-Based Exposure Limits (HBELs): Accurate determination of PDE, ADE, OEL, and F-values to safeguard human health.
- Impurity Assessment: Rigorous evaluation of impurities, extractables, and leachables (E&L) in accordance with ICH guidelines.
- Genotoxicity Risk Assessment: Comprehensive assessment of genotoxic impurities to mitigate potential risks.
- Environmental Risk Assessment (ERA): Evaluation of a product’s environmental impact.
With a proven track record and deep industry knowledge, TOXLAB is your trusted partner in navigating complex toxicological challenges.
Key services include:
- HBEL reports (PDE, ADE, OEL)
- Impurity risk assessment (ICH Q3A, Q3B, Q3C, Q3D)
- E&L risk assessment
- Genotoxicity risk assessment (ICH M7)
- F-value determination for CRP
- Environmental risk assessment
PDE Calculation and Risk Assessment Services
TOXLAB offers comprehensive PDE calculation services in strict adherence to EMA guidelines (EMA/CHMP/CVMP/SWP/169430/2012). Our team of board-certified toxicologists (DABT & ERT) possesses in-depth expertise in identifying critical effect parameters Points of Departure (POD), the No Observed Adverse Effect Level (NOAEL or NOEL), and the Lowest Observed Adverse Effect Level (LOAEL) and deriving accurate PDE/ADE values.
Our PDE reports include a detailed analysis of acute, repeat-dose, reproductive, developmental, and genotoxicity data, along with a thorough evaluation of clinical adverse effects. We effectively apply the TTC approach for genotoxic substances and address uncommon exposure routes.
Beyond PDE calculation, we support clients with PDE establishment for various substances, including cleaning agents, starting materials, and complex mixtures. Our services also encompass GMP audit support, including post-audit assistance and addressing agency queries.
Expert PDE and OEL Development
Our team comprises highly skilled toxicologists with extensive experience in developing PDE and OEL reports. All reports undergo rigorous review by American Board-Certified (DABT) and European Registered Toxicologists (ERT).
With a proven track record of delivering over 500+ PDE/ADE reports, 400+ OEL reports, and 1000+ combined reports globally, we offer unmatched expertise. Our established literature search methodology and robust quality control processes ensure accurate and reliable deliverables.
We prioritize client satisfaction by delivering reports within tight deadlines, including expedited options for urgent projects. Additionally, we can adapt to specific client templates and requirements, providing a customized service.
1. PDE Assessment
- Conduct literature reviews on drug substances
- Perform toxicological evaluations
- Calculate PDEs for active pharmaceutical ingredients (APIs)
- Develop PDE reports compliant with EMA and FDA guidelines
- Offer PDE monograph writing services
2. OEL Determination
- Evaluate occupational hazards in pharmaceutical manufacturing
- Conduct exposure assessments
- Determine OELs for APIs and pharmaceutical intermediates
- Develop comprehensive OEL reports
- Provide recommendations for exposure control measures
3. Toxicology Risk Assessment
- Perform hazard identification and characterization
- Conduct dose-response assessments
- Evaluate exposure scenarios
- Characterize risks for various pharmaceutical compounds
- Develop risk management strategies
4. Regulatory Compliance Consulting
- Assist with EMA, FDA, and other global regulatory requirements
- Help implement ICH Q3D guidelines for elemental impurities
- Advise on GMP compliance related to cross-contamination prevention
5. Training and Education
- Deliver workshops on PDE/OEL methodologies
- Offer online courses on toxicology risk assessment
- Provide customized training for pharmaceutical companies
Environmental Risk Assessment (ERA)

Environmental Risk Assessment (ERA) is a critical process to evaluate the potential impact of pharmaceuticals on the environment. By assessing a substance’s fate (how it behaves in the environment) and hazard (its potential to harm organisms), ERA helps identify and mitigate risks.
Key components of ERA include:
- Hazard identification:Determining potential adverse effects on humans or ecosystems.
- Toxicity assessment:Evaluating the harmful effects on ecological receptors.
- Exposure assessment:Estimating contact between humans or ecosystems and the contaminated environment.
- Risk characterization:Predicting the likelihood and severity of potential adverse effects.
Factors considered in ERA:
- Persistence: How long a substance remains in the environment.
- Bioaccumulation:The tendency of a substance to accumulate in organisms.
- Toxicity:The potential for a substance to cause harm.
- Predicted environmental concentration (PEC): The estimated concentration of a substance in the environment.
Regulatory frameworks:
- European Medicines Agency (EMA)
- US Food and Drug Administration (FDA)
- Australian Government
- US Environmental Protection Agency (EPA)
Focus on aquatic ecosystems: Evaluation of the Specific Endpoints
ERA for aquatic environments involves identifying receptors (species, communities, or ecosystems) and assessing their potential impacts (survival, growth, reproduction).
Comprehensive Environmental Risk Assessment (ERA)
- TOXLAB delivers detailed ERA reports that strictly adhere to regulatory standards and provide comprehensive supporting documentation.
- Our qualified toxicologists rigorously review and approve all reports.
- We employ advanced scientific methodologies to conduct tiered ERA assessments, ensuring efficient and effective evaluation. Our expertise extends to complex pharmaceuticals and novel chemical entities.
- By maintaining open communication and providing timely updates, we collaborate closely with clients to determine the appropriate level of assessment required.
- Our commitment to scientific excellence and regulatory compliance guarantees the highest quality ERA reports.
Expert ERA Services
- Our team comprises highly qualified toxicologists certified by both the American Board of Toxicology and the European Registry of Toxicologists.
- With a deep understanding of global regulatory landscapes, we deliver comprehensive ERA reports that meticulously evaluate hazards, risks, and potential environmental impacts.
- We prioritize efficiency and client satisfaction by offering rapid turnaround times and flexible service options, including 24/7 regulatory support.
- Our robust quality assurance processes ensure the highest standards of accuracy and reliability in every report.
- Trust TOXLAB for exceptional ERA expertise and unparalleled service.
TOXLAB offers a comprehensive preclinical drug development platform, combining scientific excellence with regulatory expertise. Our integrated services span from early-stage discovery to IND filing, accelerating your drug development journey.
Key capabilities include:
- Safety Assessment:Toxicology, safety pharmacology, and genotoxicity studies.
- ADME/DMPK:Absorption, distribution, metabolism, excretion, and pharmacokinetic evaluations.
- Bioanalysis:Sensitive and precise analytical methods for drug and biomarker quantification.
- Pharmacology:In vitro and in vivo efficacy studies to understand drug action.
- CMC:Chemistry, manufacturing, and controls, including formulation development, process optimization, and GMP manufacturing.
By providing a full suite of services under one roof, we streamline your drug development process, ensuring efficiency and quality.
For a detailed overview of our services, please write to us at connect@toxlab.co
Our Advantages: Global Approach
- Regional Expertise
- We developed knowledge of region-specific regulations (e.g., EMA, FDA, PMDA, NMPA)
- Having partners with local expertise in key pharmaceutical markets
- Multi-lingual Services
- Offer reports and consultations in multiple languages
- Provide translation services for PDE/OEL documentation
- Global Partnerships
- Having Collaborations with CROs and pharmaceutical companies worldwide
- Having alliances with toxicology experts in different countries
- Remote Consulting
- Provide virtual consultations and project management
- Can Provide 24/7 support to accommodate different time zones
- Cross-border Projects
- Manage multi-national assessment projects for global pharmaceutical companies
- Offer harmonized approaches for companies operating in multiple jurisdictions
